Hyperthermia: a Potent Enhancer of Radiotherapy
Clinical Oncology (2007) 19: 418-426
doi:10.1016/j.clon.2007.03.015
M. R. Horsman, J. Overgaard
Department of Experimental Clinical Oncology, Aarhus University Hospital, Aarhus, Denmark
ABSTRACT
Hyperthermia is generally regarded as an experimental treatment with no realistic future in clinical cancer therapy. This is totally wrong. Although the role of hyperthermia alone as a cancer treatment may be limited, there is extensive pre- clinical data showing that in combination with radiation it is one of the most effective radiation sensitisers known. Moreover, there are a number of large randomised clinical trials in a variety of tumour types that clearly show the potential of hyperthermia to significantly improve both local tumour control and survival after radiation therapy, without a significant increase in side-effects. Here we review the pre-clinical rationale for combining hyperthermia with radiation, and summarise the clinical data showing its efficacy. Horsman, M. R., Overgaard J. (2007). Clinical Oncology 19, 418-426
(c)2007 The Royal College of Radiologists. Published by Elsevier Ltd. All rights reserved.
Key words: Clinical trials, hyperthermia, pre-clinical models, radiation, radiosensitisation
Introduction and Historical Background
The use of heat to treat cancer is probably one of the oldest cancer therapies known. In fact, the first recorded application can be found in the Edwin Smith Surgical Papyrus, an Egyptian papyrus that can be dated back some 5000 years [1,2], in which a patient with breast cancer was treated with heat. Since the 17th century there have been numerous reports of tumour regressions in patients suffer- ing with infectious fever [3], and probably the most famous example illustrating the potential use of fever-induced treatment to control tumour growth was Coley’s toxin back in the 1800s [4]. The first real attempt to deliberately use hyperthermia to treat cancer was made by Westermark in 1898 [5], when he used water-circulating cisterns to treat inoperable carcinomas of the uterus with temperatures of 42-44°C. Since then, hyperthermia has been extensively investigated in both pre-clinical and clinical studies. However, despite this long history, hyperthermia is often considered as an experimental treatment with no realistic future in clinical cancer therapy. This is a totally erroneous evaluation. Although hyperthermia per se is probably only useful in palliative situations and has no role to play in the curative treatment of human tumours [6], unless extremely high thermal ablation temperatures can be achieved [7], there is definitive evidence that when hyperthermia is combined with other treatments significant improvements in clinical outcome are possible. This is especially true for the combination of heat and radiation [8], and in fact hyperthermia is probably one of the most effective radiation sensitisers known.
Combining Heat and Radiation in vitro and in vivo
It has been shown in vitro that the enhancement of radiation damage by heat generally occurs in all cell types regardless of whether they are neoplastic or normal [9]. This interaction between heat and radiation is dependent on a number of factors. These include heating temperature, heating time, and sequence and time interval between the two modalities, as illustrated in Fig. 1. Generally, the higher the temperature used the larger the effect seen. With exposure time there is a similar increase with increased heating, although a saturation effect can sometimes be seen [9]. Figure 1 also shows the influence of sequence and timing between the radiation and heat treatment. It is a controversial issue as to whether irradiating immediately before or after heating is superior and this is clearly illustrated in Fig. 1, in which irradiating before heating is best for HA-1 cells, whereas for EMT6 the reverse schedule is better. The consensus is that irradiating during the heating period maximises cell killing [11] and that as the interval between heat and radiation increases, regardless of sequence, the radiosensitisation by heat is rapidly lost (Fig. 1). The exact mechanism by which heat sensitises cells to radiation is not known, but most evidence suggests that heat primarily interferes with the cells’ ability to deal with radiation-induced DNA damage [12,13].
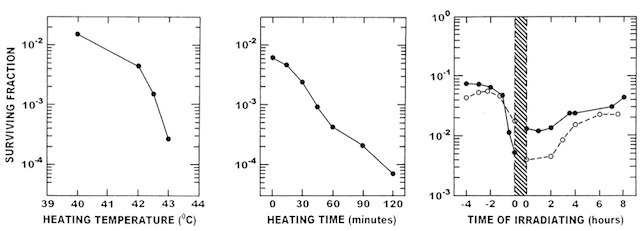
Fig. 1 – Left panel: survival of Chinese hamster cells exposed to different heat temperatures for 2 h and irradiated with 12 Gy in the middle of the heating period. Middle panel: response of HA-1 cells to irradiation with 12 Gy and then immediately heated at 43°C for different times. Right panel: effect of heat (43°C; 60 min) and radiation (6 Gy) on the response of EMT-6 (◦) or HA-1 (•) cells. The heating period is shown by the shaded area, with the irradiation given at different times before or after heating. Figures redrawn from [9,10].
In vivo the situation is slightly more complicated, with the heat-induced enhancement of radiation damage generally considered to be the result of two mechanisms. One is a direct radiosensitisation, as seen in vitro. However, in tumours there is an additional indirect mechanism that results from the heat killing the radioresistant hypoxic cell population. A tumour’s vascular supply is structurally and functionally abnormal when compared with that of the normal tissue from which it is derived [14], and as a result is unable to meet the oxygen and nutrient demands of the growing tumour mass. This results in the development of areas that are nutrient deprived, low in oxygen, and highly acidic [14]. Cells that survive in these adverse conditions are often referred to as hypoxic and have not only been identified in both animal [15] and human [14] solid tumours, but are also known to have a significant negative effect on the tumour response to radiation therapy [16]. Several in vitro studies have reported that cells under hypoxic conditions are more sensitive to the lethal effects of hyperthermia than cells in a well-oxygenated environment [17e19]. Under well-defined nutrient conditions, acute hypoxia alone does not have any significant influence on the cellular response to hyperthermia [19e21]. However, prolonged oxygen deprivation or chronic hypoxia will increase cellular heat sensitivity [19,22,23]. Prolonged hypoxia generally leads to metabolic changes, which in turn alter several other parameters, such as acidity, and it is these changes that are responsible for the increased sensitisation to hyperthermia [17,20,22,24].
Factors Influencing the Response to Single Treatments
The difference between the enhancement of the radiation response via a direct heat radiosensitisation or hyperthermic cytotoxicity is illustrated in Fig. 2. Generally, for tumours the thermal enhancement of the radiation response is greatest when the radiation and heat are administered simultaneously (i.e. the time when hyperthermic radio- sensitisation is probably greatest) and decreases with the introduction of an interval between the heat and radiation. This reduction typically reaches a nadir at around 4 h when heat is administered after irradiation (i.e. the time when hyperthermic cytoxicity predominates), but may take slightly longer when the reverse schedule is used. Clearly, there are some tumour models shown in Fig. 2 that do not seem to show these time-dependent differences. However, this may simply reflect the fact that in those studies a truly simultaneous heat and radiation were not given, as was the case for the two studies that showed the greatest enhance- ment [27,28]. There is also evidence that even when irradiating immediately before or after heating there is a significant reduction in the enhancement obtained compared with that observed when the radiation is given in the middle of the heating period [6].
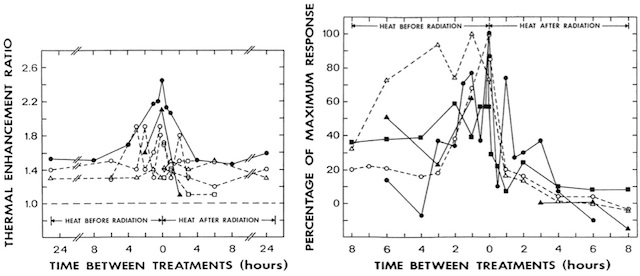
Fig. 2 – Left panel: thermal enhancement ratios (ratio of the radiation doses for radiation alone and radiation plus heat to produce the same effect) for different murine tumour models as a function of the time interval and sequence between heating (42.5°C; 60 min) and irradiation. The results are for mammary carcinomas (●▲), carcinoma NT (▵), SQ carcinoma (○), sarcomas Fa (○), F (▽), and S (□). Right panel: thermal enhancement ratios (normalised to a percentage of the maximum effect) for different murine normal tissues as a function of the time interval and sequence between heating for 60 min at different temperatures and irradiation. The results are for intestine at 41.8°C (●), ear skin at 42°C (○) and 43°C (▵), foot skin at 42.5°C (▲), and cartilage at 43°C (■). Figures redrawn from [25,26].
Also illustrated in Fig. 2 is the effect of scheduling and timing on the response of various normal tissues to the combination of heat and radiation. As for the tumour, a simultaneous application gives rise to the greatest enhancement and as the time interval between irradiating and subsequent heating is increased so the enhancement decreases, almost completely disappearing with a 4h interval. When heating is administered before irradiation, there is also a reduced enhancement, although the effect seems to be persistent as the time interval increases and is certainly still present with a 4e6 h interval. The fact that there is some thermal enhancement of radiation damage in tumours when heat is administered 4 h after irradiating, but little or no effect in normal tissues, is additional confirmation that with this time interval and schedule the effect in tumours is primarily due to hyperthermia killing radiation-resistant hypoxic cells, because unlike tumours most normal tissues are devoid of any significant hypoxia [14]. The presence of a somewhat larger enhancement when the heat is administered before radiation may be indicative of a persistent thermosensitisation mechanism.
Regardless of whether the combination of radiation and heat is given in a simultaneous or sequential schedule, the thermal enhancement of both tumours and normal tissues will be dependent on the heating time and temperature. This is illustrated in Fig. 3. For a simultaneous treatment, the relationship is very simple, in that the higher the temperature and the longer the heating time, then the larger the thermal enhancement, and this is true for both tumours and normal tissues. As such, no therapeutic advantage is to be expected from a simultaneous treatment if the tumour and normal tissue are heated at the same temperature. This is not the situation with a sequential treatment. In tumours there is an enhanced response with increasing temperature and time, but as the temperature increases so the degree of difference diminishes and eventually a saturation level is reached. Although this plateau is at a relatively low level when compared with the enhancements seen with a simultaneous treatment, the fact that a sequential treatment has little or no effect in normal tissues means that there is a substantial therapeutic benefit.
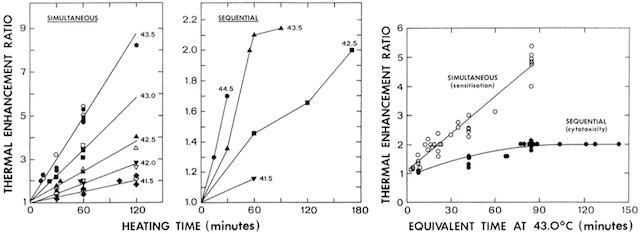
Fig. 3 – Left and centre panels: influence of heating time and temperature on the thermal enhancement ratio when heat and radiation are combined in either a simultaneous or sequential (heat 4 h after irradiating) protocol. Thermal enhancement ratios were determined from the ratios of the radiation doses for radiation alone and radiation plus heat to produce the same effect in C3H mammary carcinomas (closed symbols) or normal skin (open symbols) at the temperatures indicated. Right panel: thermal enhancement ratios for simultaneous or sequential heat and radiation treatments in tumours converted to an equivalent time at 43°C. Figures redrawn from [8,29].
Clinically Relevant Issues with Thermoradiosensitisation
The combination of heat and radiation in a clinical regimen will probably be in a fractionated schedule. It has been shown from studies in vitro, and with both tumours and normal tissues in vivo, that hyperthermia may induce a temporary resistance to a subsequent heat treatment [30], a phenomenon often referred to as thermotolerance. The kinetics and degree of thermotolerance that develops is dependent on the cell type, the heating temperature, the time of heating, and the interval between successive heat treatments [30]. However, generally there is a lag phase that can be of a few hours’ duration before thermo- tolerance begins to manifest itself, after which thermo- tolerance increases, reaching a maximal effect within the first 24 h, and then decays. This decay can take anywhere from a few hours to several days before completely disappearing, depending on how quickly and to what degree the maximal effect occurs [30].
Thermotolerance can also affect the response to the combination of heat and radiation, regardless of whether a simultaneous or sequential schedule is used. Pre-heating a tumour and then subsequently irradiating and then simultaneously or sequentially re-heating, reduces the thermal enhancement compared with that seen without any pre-treatment (Fig. 4). Interestingly, the degree of ‘relative resistance’ in this example follows the same time course as seen with the development of thermotolerance for heat alone [30]. The role of thermotolerance when multifractional heat and radiation treatments are used is less clear. One pre-clinical study reported that if hyperthermia and radiation were combined in a fractionated schedule of five daily fractions, then the observed thermal enhancement was not significantly different from that obtained with a single fraction, regardless of whether the heat was administered simultaneously with radiation or 4 h later in a sequential regimen [33]. If the interval between fractions was extended to allow for thermotolerance to disappear, then the actual thermal sensitisation increased above that obtained with a single dose, reaching a maximum when a 5-day interval was used. This should not be considered as an improved response, because although the thermal sensitisation had increased, the response to radiation alone with a 5-day interval was actually reduced. These results contrast with those from another study, in which thermal sensitisation was reduced by giving one or four pre-treatments with hyperthermia [34]. In skin, the effect was unchanged when heat and radiation were given in two or five fractions, regardless of whether the heat followed immediately after irradiating or 3 h later [34,35]. Similar results were seen with fractionated heat and radiation in mouse ear, provided the heat was administered after irradiating [36]. If the heat was applied before the radiation in each combined treatment, a large reduction in thermal sensitisation was observed with increasing number of fractions.
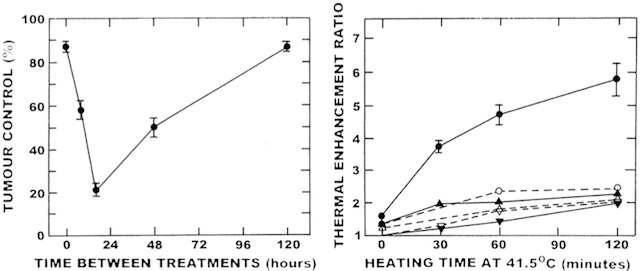
Fig. 4 – Left panel: percentage local tumour control in C3H mammary carcinomas that were heated (43.5°C; 30 min) at various time intervals before a simultaneous treatment with radiation (15 Gy) and heat (43.5°C, 60 min). Tumours at the 0 h interval received no prior heat before the simultaneous treatment. Right panel: the effect of step-up heating (SUH; 41.5°C for various times + 44.5°C for 10 min) or step-down heating (SDH; 44.5°C for 10 min + 41.5°C for various times) on the thermal enhancement ratios (ratio of the radiation doses for radiation alone and radiation plus heat to produce the same effect) in C3H mammary carcinomas (closed symbols) and normal skin (open symbols). The results are for radiation with single heating (▼▽), SUH (▲△), and SDH (●○). Errors on all figures are ±1 standard error. Figures redrawn from [31,32].
Another clinical problem that needs to be considered is that during heat treatment considerable fluctuations in tissue temperature are often observed [9,37]. Experimentally this phenomenon has been examined by subjecting tissues to two consecutive heat treatments, one higher than the other. Exposure to a higher temperature followed by a lower is referred to as step-down heating (SDH), whereas the reverse treatment order is called step-up heating (SUH). From the data accumulated to date, it has been found that with SUH the tumour response is the result of an additive effect of the two heat treatments, whereas with SDH not only is there additional cell killing from the higher temperature, but significant sensitisation to the second heat treatment is also observed [38]. The effects of SDH and SUH on a combined heat and radiation treatment have been studied both in vitro [39-42] and in tumours and normal tissues in vivo [31,43,44]. Examples of the in vivo effects are summarised in Fig. 4. In both tissues, SUH produced only a small increase in radiation response above that found with a single heat treatment. A slightly larger enhancement was obtained with SDH in skin, but the thermal enhancement was much less than that found in tumours, suggesting that the phenomenon of SDH may actually be beneficial. These thermal enhancements produced by both SDH and SUH when combined with a simultaneous heat and radiation treatment are substantially reduced with the introduction of a time interval between irradiating and heating, but are still larger than that seen with heat and radiation alone [44].
Role of the Tumour Vasculature
Another factor that plays a critical role in influencing the tissue response to heat, and thus probably also has an important effect on thermal sensitisation, is blood flow. Blood flow is one of the major vehicles by which heat is dissipated. Thus, the vascular supply will influence the ability to heat the tissue. The relationship between blood flow and heating has been examined [45,46], and in general the lower the rate of blood flow the easier it is to heat the tissue. Blood flow is also important in determining the type of microenvironment that exists in tumours and thus sensitivity to heat; the compromised tumour blood flow results in the development of adverse microenvironmental conditions [14] and cells existing in such adverse conditions are more sensitive to hyperthermia [17-24]. There is now clear in vivo evidence that modifying tumour blood flow using physical clamping [47-49], physiological modifiers [50-52], or vascular disrupting agents (VDAs) [53,54], can enhance the tumour response to heat and that this involves both a better heating and increased sensitivity.
The vascular supply to a tumour can also play a role in the ability of heat to increase the effect of radiation. This is illustrated by the fact that larger tumours show a greater enhancement of radiation damage by heat than smaller tumours, although this is only when radiation and heat are given in a sequential schedule, not with a simultaneous treatment [37]; larger tumours are generally less well perfused than smaller tumours and as a result contain a higher proportion of cells that are radiation resistant, yet heat sensitive [14]. Thus, the increased thermal radiosen- sitivity seen in larger tumours is simply the result of an increase in heat cytotoxicity. Supportive data for this come from the finding that the greater the hypoxic fraction in tumours the greater the thermal enhancement [55], and that tumour sensitivity to heat alone also increases with size [56,57].
Another vascular-mediated effect that may play a role in thermoradiosensitisation is related not to any influence of the tumour vascular supply on heat, but rather to the effect of heat on the tumour vasculature. It is well known that heating can change tumour blood flow, but the effect depends on the thermal dose; at high thermal doses blood flow decreases and at low thermal doses it increases [58,59]. This will naturally affect tumour oxygenation status and several studies have investigated this issue in animal tumours and human tumour xenografts [60-62]. The consensus is that the heat-induced changes in tumour oxygenation parallel the effects on tumour blood flow, namely that at high thermal doses tumour oxygenation decreases and at low thermal doses it increases. However, the influence this has on the tumour radiation response is controversial. At high temperatures, the decrease in flow and associated reduced oxygenation are severe and main- tained for periods that are long enough to induce substantial cell killing [63]. The cells most likely to die first are those that are already hypoxic and this may be an alternative and indirect method by which hyperthermic cytotoxicity can occur in vivo. With more mild hyperthermia treatments, any increase in tumour oxygenation will naturally decrease the radioresistant population and tumours will therefore be more radiation sensitive. As this effect occurs rapidly after starting to heat [62], it may explain some of the thermo- radiosensitisation with a simultaneous treatment. The controversy comes with the duration of this improved effect, with some studies reporting a rapid return to normal oxygenation status in tumours when heating stops [62], whereas others suggest that the effect is maintained for up to 24 h [64]. If this latter is true, it could sensitise tumours to subsequent fractionated radiation treatments. However, persistent improvements in oxygenation status after mild hyperthermia treatments are difficult to explain because the physiological changes that probably account for the enhanced tumour oxygenation by such heat treatments should return to normal at or soon after the cessation of heating.
The importance of the tumour vascular supply for thermal radiosensitisation suggests that modifying blood flow could be used to further improve the therapeutic potential. This has been shown with VDAs, many of which are currently in clinical testing [65]. When combining VDAs with thermoradiation, timing and sequencing of the drug, heat and radiation treatments will be critical to the response obtained. Additional studies have shown that when VDAs and radiation are combined, timing is not so important provided the VDA is given after irradiating [53]; giving the VDAs before radiation induces hypoxia that can actually reduce the efficacy of the subsequent radiation treatment. For the combination of VDAs with heat, the greatest enhancements are found when the heat is applied after drug injection, at the time when the drug-induced vascular collapse is maximal, which is typically 1e6h depending on the VDA used [53,54]. This would argue for giving the VDAs in the middle of a sequential radiation and heat treatment, and in fact such a schedule has been used with a number of different VDAs [53,54]. Representative results for one tumour model and a normal tissue are shown in Fig. 5. In the tumour, hyperthermia alone only begins to enhance the radiation response at temperatures above 41.5°C, and this enhancement becomes larger as the temperature increases. With the VDAs there is an effect of the drugs alone on radiation, but when combined with thermoradiation the radiation response is enhanced at temperatures as low as 40.5°C. In fact, at this temperature the thermal enhancement is as good as one finds with 43°C alone. An even greater thermal enhancement occurs with the VDA and heat as the temperature increases, and it is interesting to speculate as to what enhancements could be achieved with temperatures above 41.5°C. Also shown in this figure are the thermal enhancements obtained with the VDA, heat and radiation combination in normal foot skin. Neither the VDAs nor heating alone had any effect on the radiation response. More importantly, the combination of VDAs with heat also had no influence on the radiation response, at least at a temperature of 41.5°C. This means that the thermal enhancement of the tumour response at this particular temperature is a pure therapeutic benefit.
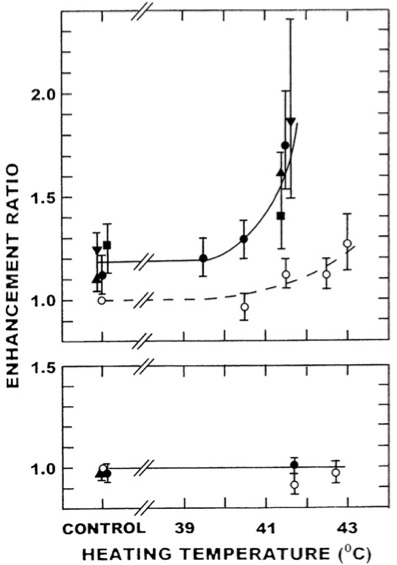 Fig. 5 – The effect of combining radiation, vascular disrupting agents (VDAs) and heat on local control in a C3H mammary carcinoma (upper panel) and skin damage in CDF1 mice (lower panel). Tumours or skin were locally irradiated 4 h before heating at the indicated temperatures for 60 min (B), or mice intraper- itoneally injected with either 25 mg/kg CA4DP (▲), 50 mg/kg Oxi4503 (■), 20 mg/kg DMXAA (●), or 150 mg/kg FAA (▼) 60 min after irradiating and then heated 3 h later. Enhancement ratios (with 95% confidence intervals) represent ratios of the radiation doses for radiation alone and radiation with VDA/heat to produce the same effect. Figures redrawn from [53,54] and also include unpublished observations.
Fig. 5 – The effect of combining radiation, vascular disrupting agents (VDAs) and heat on local control in a C3H mammary carcinoma (upper panel) and skin damage in CDF1 mice (lower panel). Tumours or skin were locally irradiated 4 h before heating at the indicated temperatures for 60 min (B), or mice intraper- itoneally injected with either 25 mg/kg CA4DP (▲), 50 mg/kg Oxi4503 (■), 20 mg/kg DMXAA (●), or 150 mg/kg FAA (▼) 60 min after irradiating and then heated 3 h later. Enhancement ratios (with 95% confidence intervals) represent ratios of the radiation doses for radiation alone and radiation with VDA/heat to produce the same effect. Figures redrawn from [53,54] and also include unpublished observations.
Clinical Trials with Heat and Radiation
The large body of pre-clinical data clearly showing the benefit of combining radiation and hyperthermia has resulted in the translation of this approach into a number of clinical trials. A meta-analysis of all published trials, in which patients were randomised to radiation or radiation and heat, are summarised in Table 1. These results show locoregional control, which is the most relevant end point for locally applied treatments, and show significant heat- induced improvements in a number of distinct sites, including chest wall, cervix, rectum, bladder, melanoma, and head and neck. When all these clinical results were combined (1861 patients from 23 trials), a highly significant improvement was obtained (P<0.0001), confirming the beneficial effect of combining hyperthermia with radiation. This positive result was seen despite the fact that the radiation and heat treatments were highly variable among the various tumour types. A number of these clinical studies reported on the potential of combining radiation and hyperthermia to improve overall survival [66-71]. In two trials in head and neck cancer [66,67] and one involving tumours of the pelvic region [70], significant improvements in survival were observed in the radiation and heat-treated patients compared with radiation alone. For the head and neck trials, tumour stage may have been an important factor influencing the response, as in one study a survival benefit of adding heat was only seen in the stage III and IV patients [66], whereas in the other study where a clear benefit was seen, the patients were all stage IV [67]. Tumour type may also be an important factor determining outcome, as the study investigating the effect of heat on the radiation response in tumours of the pelvic region [70] reported that the benefit seen in the patients was primarily influenced by the large enhancement in the cervix group; in rectum and bladder no significant improvements were seen. For the remaining trials, no survival benefit was found for radiation and heat compared with radiation alone [68,69,71]. In one of those studies, using a variety of recurrent or persistent tumours [68], there was also no significant improvement in local control by adding heat to the radiation treatment.
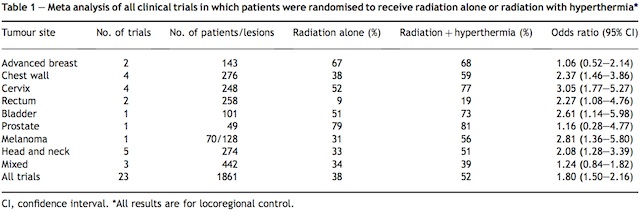
This lack of benefit in both local control and survival was probably because of the 88 patients who received heat only one actually received an adequate hyperthermia session. Another study in rectal carcinoma [71] did report an increase in survival, but this was not significant, which may simply have reflected the low patient number (20 patients in each arm). The most surprising negative result was that from the large breast cancer study that reported results from five randomised trials, and had shown a clear benefit of hyperthermia on local control [69]. However, the lack of any improvement in overall survival was attributed to the finding that many of the patients had a history of metastatic disease outside the treatment area at the time of randomisation. Furthermore, most of the patients also showed progression outside the treatment area during follow-up, which would clearly affect survival.
Overall, the randomised clinical trials clearly showed significant improvements in local tumour control by combining heat with radiation, and in certain tumour types these effects could be translated into enhanced overall survival. Such benefits are only relevant provided there are also no significant increases in morbidity. A number of these clinical studies did investigate the possible influence of heat on early and late adverse reactions induced by radiation. None of the studies found any significant increase in acute toxicity [66-72], and although one study did report a slight increase in late damage [67], no increase in late reactions was seen in any of the other studies [66,68-72].
Conclusions and Future Perspectives
Despite the extensive pre-clinical studies establishing the rationale for combining heat with radiation, and the large number of randomised clinical trials showing a significant benefit of adding heat treatments to radiation therapy to improve outcome, the use of this combined therapy approach has not been adopted in routine clinical practice. This can probably be attributed to the difficulties in adequately heating tumours in patients. The consensus was generally that all one required to effectively enhance the radiation response was a few good heatings. This is probably still true today, although there are suggestions that mild hyperthermia treatments can also be effective. However, the role of mild hyperthermia in improving the radiation response is still not proven. If it were true then one would probably have expected many more trials to be successful. Those trials that did successfully combine heat and radiation were those in which a few good heatings were obtained, and to achieve that one clearly needs good equipment and dedicated personnel.
So, how can we change the current thinking about the application of hyperthermia and radiation? Clearly, with so many randomised trials showing obvious benefits it is unlikely that carrying out additional trials would be the answer. What is needed are improvements in the physics and biology so that preferential tumour heating becomes easier. From a biological standpoint, there are numerous studies demonstrating that the application of clinically relevant vascular modifying drugs not only improve tumour heating, but can also enhance the efficacy of heat; this latter effect will make an easily obtainable mild hyper- thermia treatment equivalent to a more difficult to achieve, yet more effective, higher temperature. Advances in heating technology are another area where improve- ments in heating can be achieved, and there is certainly considerable interest and effort being made in improving current approaches and developing new methods. Obvi- ously, the more we do the better it will become. However, whatever the outcome we should always remember that despite the scepticism often shown by certain groups in the scientific community, hyperthermia is a very effective sensitiser of radiation and clinically it works, which is probably the most important issue that concerns cancer patients.
Acknowledgement. This publication was made possible through financial support from the Danish Cancer Society.
Author for correspondence: M. R. Horsman, Department of Experimental Clinical Oncology, Aarhus University Hospital, Nørrebrogade 44, Bldg. 5, DK-8000 Aarhus C, Denmark. Tel: þ45- 89492622; Fax: þ45-86197109; E-mail: mike@oncology.dk
Received 8 March 2007; accepted 27 March 2007

